Background:
Click for full size
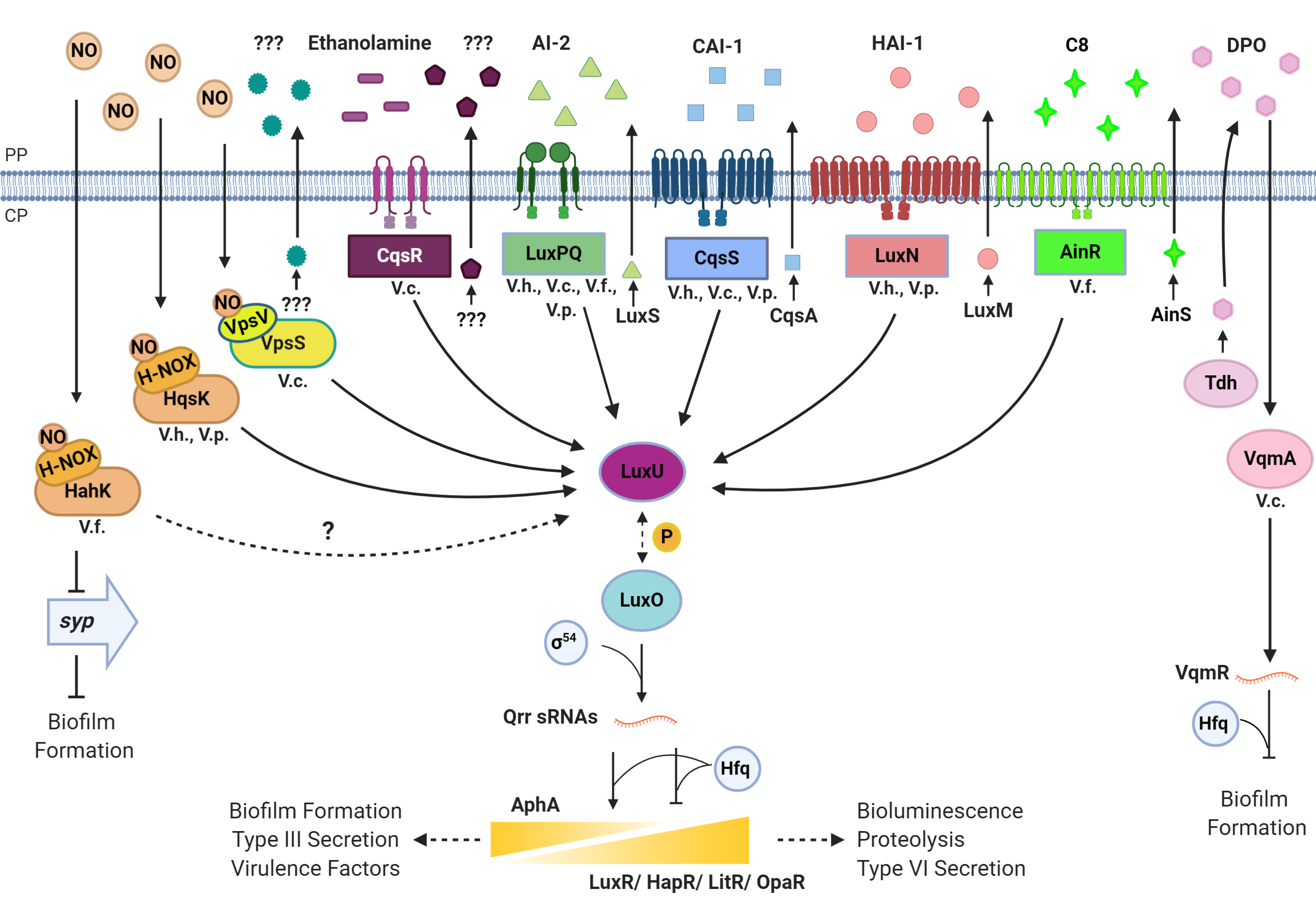
Quorum sensing relies on extracellular signaling molecules called autoinducers (AIs). As a population of quorum-sensing bacteria grows, the extracellular concentration of AIs increases. When a signal threshold is reached, the group responds with a population-wide alteration in gene expression. Our lab studies quorum sensing in the model organism Vibrio harveyi. V. harveyi makes and responds to three AIs that enable three modes of communication: intraspecies, intragenus, and interspecies. At low cell density (LCD), the extracellular AI concentration is low. The three membrane-bound sensors begin a phosphotransfer cascade that results in phosphorylation of the response regulator LuxO. LuxO~P initiates transcription of five genes encoding small noncoding RNAs (sRNAs), called the quorum regulatory RNAs (Qrr1-5). The Qrr sRNAs repress translation of the master quorum-sensing transcription factor LuxR and stimulate translation of AphA, the LCD master transcription factor. At LCD, AphA levels are high and LuxR levels are low. As cells grow and divide, the extracellular concentration of AIs increases proportionally in the surrounding environment. At high cell density (HCD), the concentration of AIs is also high. AI binding to the membrane-bound receptors reverses the phosphorylation cascade, which terminates transcription of the Qrr sRNAs. In the absence of Qrr sRNAs, LuxR protein is produced, and AphA translation ceases. Thus, high levels of LuxR are made at HCD, while AphA is only present at LCD.
Click for full size
Projects:
Mechanisms of LuxR transcriptional regulation of quorum-sensing genes
V. harveyi LuxR is the founding member of the group of transcription factors that control quorum sensing in all vibrios. LuxR shares high amino acid identity with other LuxR proteins: 71% with HapR (Vibrio cholerae), 96% with OpaR (Vibrio parahaemolyticus), and 93% with SmcR (Vibrio vulnificus). LuxR is a unique member of the TetR family of transcription factors that both activates and represses a regulon of >600 genes through binding to a degenerate consensus DNA binding motif. There is currently a gap in existing knowledge of TetR regulatory mechanisms because LuxR is the only known TetR protein that activates transcription. We are using a combination of structural biology, biochemistry, and genetics to determine how LuxR functions as an activator and a repressor of quorum-sensing genes.
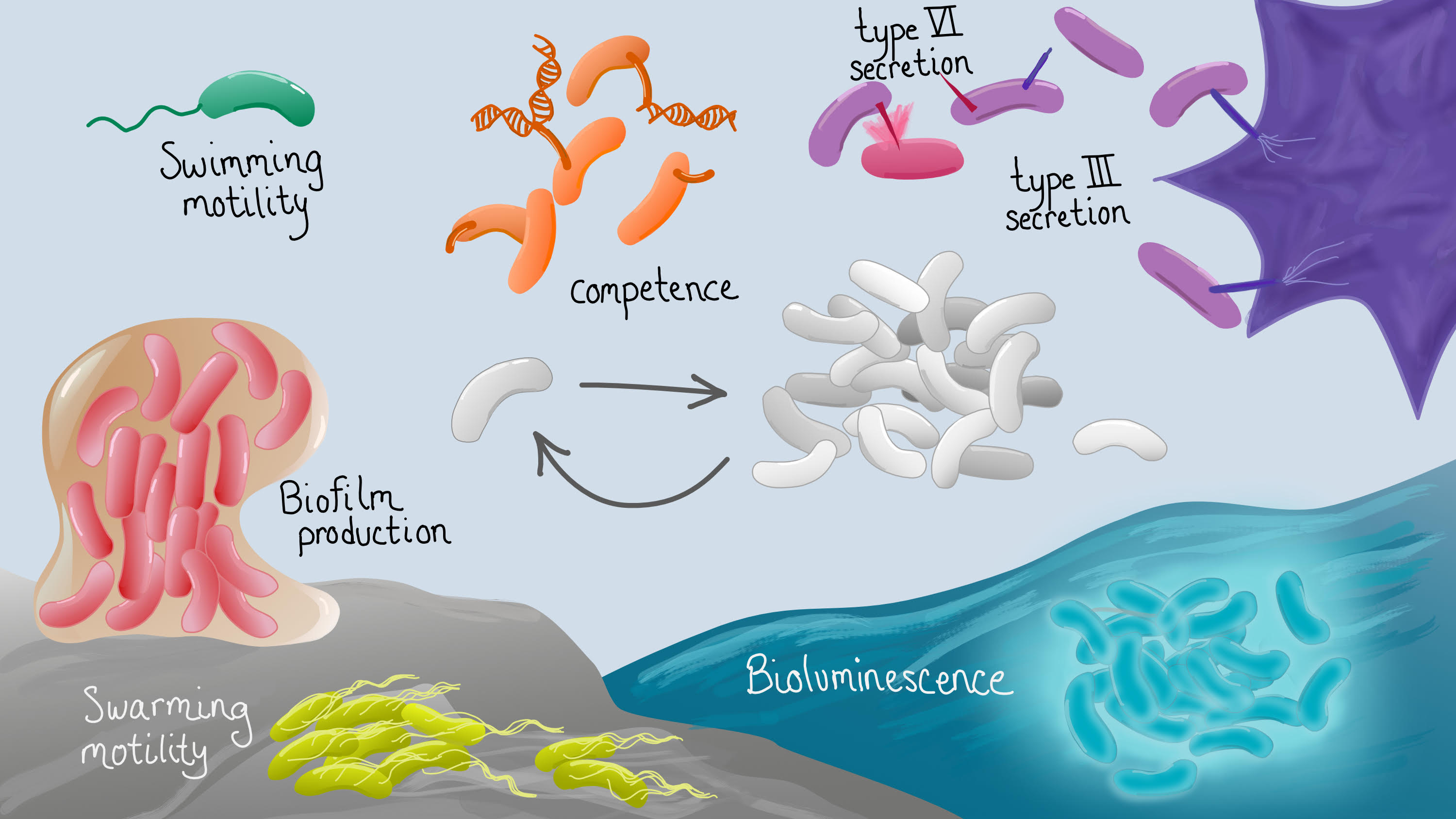
Quorum-sensing regulation of group behaviors in vibrios
Among the >600 genes controlled by quorum sensing through LuxR are genes required for type III secretion, type VI secretion, motility, and osmotic stress regulation. We are interested in the patterns of gene expression that control these important physiological outputs. LuxR, AphA, and the Qrrs are the central regulators of these expression patterns, though other downstream transcription factors also play important roles. Using bacterial genetics, bioinformatics, and biochemistry, we are examining the hierarchy of gene regulation that contributes to expression of key developmental and physiological pathways in vibrios.
Chemical inhibitors of quorum sensing in vibrios
As the central regulator of virulence in all pathogenic vibrios, LuxR is an excellent target for developing antibiotics. We have an on-going collaboration with Dr. Laura Brown (IU Chemistry) to screen for and synthesize molecules that modulate LuxR activity. We use both in vivo and in vitro assays to identify molecules and determine their mechanisms of action on LuxR proteins from V. harveyi, V. cholerae, and V. vulnificus.